PharmaJet Partner Zydus Cadila Announces Emergency Use Authorization Approval for World’s First Plasmid DNA COVID-19 Vaccine
Error message
- Notice: Undefined property: stdClass::$ds_changed in eval() (line 16 of /var/www/7/modules/php/php.module(80) : eval()'d code).
- Notice: Undefined property: stdClass::$ss_search_api_url in eval() (line 20 of /var/www/7/modules/php/php.module(80) : eval()'d code).
- Notice: Undefined property: stdClass::$tm_title in eval() (line 20 of /var/www/7/modules/php/php.module(80) : eval()'d code).


PharmaJet , the maker of innovative, needle-free injection technology, today announced that its partner, Zydus Cadila , has received the Emergency Use Authorization (EUA) from the Drug Controller General of India (DCGI) for ZyCoV-D the worlds first Plasmid DNA Vaccine for COVID-19.
PharmaJet®, the maker of innovative, needle-free injection technology, today announced that its partner, Zydus Cadila, has received the Emergency Use Authorization (EUA) from the Drug Controller General of India (DCGI) for ZyCoV-D the world’s first Plasmid DNA Vaccine for COVID-19. The vaccine, ZyCoV-D, is exclusively administered using the PharmaJet Tropis® Needle-free Injection System.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210823005175/en/
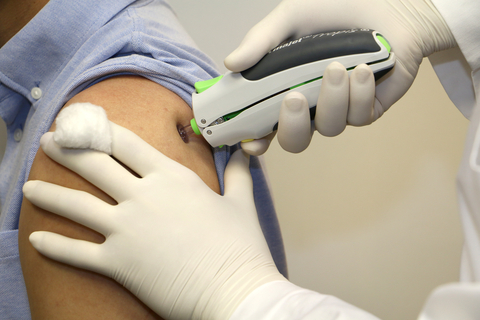
The COVID-19 vaccine is exclusively administered using the PharmaJet Tropis Needle-free Injection System. (Photo: Business Wire)
Speaking on this development, Mr. Pankaj R. Patel, Chairman, Cadila Healthcare Ltd., said, “This is an historic milestone with ZyCoV-D, a product of Indian innovation becoming the world’s first DNA vaccine being offered for human use and supporting the world’s largest immunization drive. We are particularly happy that our vaccine will contribute to this fight against COVID-19 and enable the country to vaccinate a larger population especially in the age group of 12-18 years. I would like to thank all the researchers, clinical trial investigators, volunteers and the regulators who have supported this endeavor.”
The DCGI decision is based on data from a pivotal phase 3 clinical trial which enrolled over 28,000 volunteers. Included in the trial were adolescents in the 12-18 age group, a first for a COVID-19 vaccine trial in India. ZyCoV-D exhibited robust immunogenicity, tolerability, and safety.
Chris Cappello, President and CEO, PharmaJet, said, “We congratulate our partner Zydus Cadila for achieving this critical milestone. The Emergency Use Authorization (EUA) is a huge step forward to help in the eradication of COVID-19. It demonstrates the power of DNA-based vaccines and broadens the use of needle-free jet injection. We are proud to be part of the development and commercialization of the first ever plasmid-based DNA vaccine.”
For more information about PharmaJet visit www.pharmajet.com.
Refer to Instructions for Use to ensure safe injections and to review risks.
1 Data on file
About PharmaJet
PharmaJet’s mission is to improve people’s lives through needle-free technology. PharmaJet Needle-free Systems provide increased vaccine effectiveness, a preferred patient and caregiver experience, and a proven path to commercialization. They are also safe, fast, and easy-to-use. The Stratis® System has U.S. FDA 510(k) marketing clearance, CE Mark, and WHO PQS certification to deliver medications and vaccines either intramuscularly or subcutaneously. The Tropis® System has CE Mark and WHO PQS certification for intradermal injections. For more information visit www.pharmajet.com.
Zydus’ Vaccine research program
The Vaccine Technology Centre of Zydus Cadila has a wide range of capabilities in developing and manufacturing viral, toxoid, polysaccharide, conjugate, and other subunit vaccines for unmet needs. In fact, Zydus was the first company in India to develop and indigenously manufacture the vaccine to combat Swine Flu during the pandemic in 2010. In the past, it has also indigenously developed numerous vaccines successfully including tetravalent seasonal influenza vaccine (first company in India to indigenously develop and commercialize), Inactivated Rabies vaccine (WHO Prequalified), Varicella vaccine (first Indian company to indigenously develop and receive market authorization), Measles containing vaccines (MR, MMR, Measles), Typhoid conjugate vaccine, pentavalent vaccine (DPT-HepB-Hib) etc. to name a few. The company also has a strong pipeline of vaccines like Measles-Mumps-Rubella-Varicella (MMRV), Human papillomavirus vaccine, Hepatitis A, Hepatitis E vaccines which are at various stages of development. For more information, visit https://zyduscadila.com/
View source version on businesswire.com: https://www.businesswire.com/news/home/20210823005175/en/
