Vera Therapeutics Presents Positive 72-Week Data Showing eGFR Stabilization in the Phase 2b ORIGIN Clinical Trial OLE in IgA Nephropathy
BRISBANE, Calif., Jan. 25, 2024 (GLOBE NEWSWIRE) -- Vera Therapeutics, Inc. (Nasdaq: VERA), a late clinical-stage biotechnology company developing and commercializing transformative treatments for patients with serious immunologic diseases, today announced positive 72-week data from the open label extension (OLE) period of its Phase 2b ORIGIN clinical trial of atacicept in participants with IgA nephropathy (IgAN). In aggregate, the 72-week data with atacicept are consistent with a profile of true disease modification in IgAN.
– Participants treated with atacicept for 72 weeks showed consistent and sustained reductions in Gd-IgA1, hematuria, and UPCR, with stable eGFR over the duration of treatment
– Placebo cohort participants who crossed over to atacicept 150 mg in the OLE had similar outcomes at 72 weeks as atacicept cohort in the first 36 weeks of the trial
– Atacicept was generally well-tolerated in the OLE period of the trial, consistent with the randomized period
– 72-week data provide additional confidence in the ongoing pivotal Phase 3 ORIGIN 3 clinical trial
BRISBANE, Calif., Jan. 25, 2024 (GLOBE NEWSWIRE) -- Vera Therapeutics, Inc. (Nasdaq: VERA), a late clinical-stage biotechnology company developing and commercializing transformative treatments for patients with serious immunologic diseases, today announced positive 72-week data from the open label extension (OLE) period of its Phase 2b ORIGIN clinical trial of atacicept in participants with IgA nephropathy (IgAN). In aggregate, the 72-week data with atacicept are consistent with a profile of true disease modification in IgAN.
“Data from the OLE show the consistent and sustained reductions of Gd-lgA1, hematuria, and UPCR, as well as the stability of eGFR over 72 weeks in participants with IgAN. The demonstration of stable eGFR well beyond a year in participants receiving atacicept represents an important potential advancement for IgAN patients and has potential implications for the future treatment paradigm in this disease. It is also exciting that the data from the OLE show that switching to atacicept halted the eGFR decline seen in participants initially treated with placebo, with similar reductions in Gd-IgA1, hematuria, and UPCR as shown in the active cohort in the first 36 weeks,” stated Richard Lafayette, M.D., F.A.C.P., Professor of Medicine, Nephrology and Director of the Stanford Glomerular Disease Center at Stanford University Medical Center.
“We are thrilled to present this package of positive new data from the OLE of the Phase 2b ORIGIN clinical trial during our R&D Day, which will be held today in New York. We believe these data further support our belief that atacicept has the disruptive potential to stand out as a disease-modifying treatment for patients with IgAN,” said Marshall Fordyce, M.D., Chief Executive Officer of Vera Therapeutics. “This is an exciting period for Vera as the integrated data package for atacicept continues to mature. Importantly, the ongoing pivotal ORIGIN 3 trial is well underway, with enrollment on track to be completed in the second half of this year.”
After completing the 36-week randomized, double-blind, placebo-controlled period of the Phase 2b ORIGIN trial, all participants were eligible to receive atacicept 150 mg in the OLE. Of the 116 randomized participants, 106 completed 72 weeks.
Participants treated with atacicept for 72 weeks demonstrated a 62% reduction in Gd-IgA1, a reduction in the percentage of participants with hematuria to 19%, and a 48% reduction in UPCR in the per-protocol (PP) analysis. Importantly, participants had consistent and stable eGFR with 0 mL/min/1.73m2 change from baseline at 72 weeks. Of note, it has been shown that eGFR declines by approximately 1 mL/min/1.73m2 per year in the general population (Baba M, et al. PLOS ONE 2015).
Atacicept 72-week Data From the Phase 2b ORIGIN Trial Are Consistent With a Profile of True Disease Modification
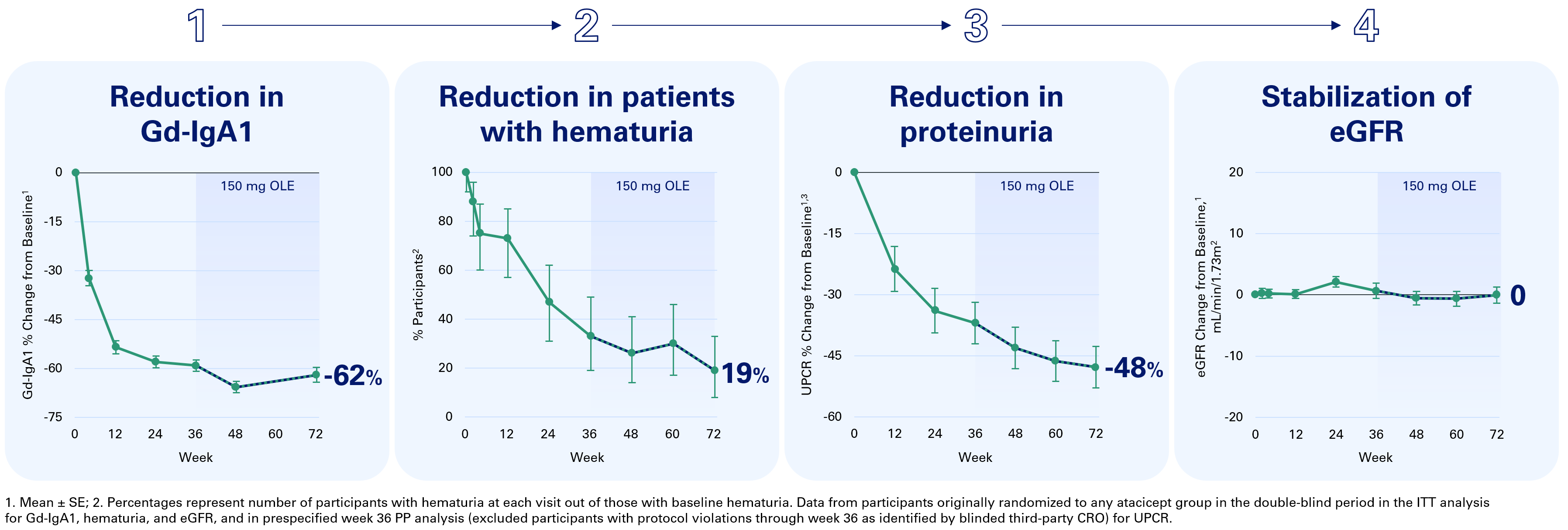
Participants who switched from placebo to atacicept demonstrated similar outcomes across each of the key indicators of IgAN as compared to participants originally randomized to atacicept during the first 36 weeks of the trial, including a 59% reduction in Gd-IgA1, a reduction in the percentage of participants with hematuria to 41%, and a 47% reduction in UPCR in the PP analysis. In addition, eGFR stabilization was observed in participants who switched from placebo to atacicept with a -3.2 mL/min/1.73m2 change from baseline at 72 weeks compared to -4.9 mL/min/1.73m2 at 36 weeks.
Safety data in the OLE were consistent with the randomized period and indicated that atacicept was generally well-tolerated. These data build upon the prior experience of atacicept in randomized, double-blind, placebo-controlled clinical trials in over 1,500 participants to date across different indications – in which atacicept was generally well-tolerated.
The R&D Day presentation, which includes the OLE 72-Week data slides and commentary, will be available on the Company’s website at the Investor Calendar.
About the Phase 2b ORIGIN clinical trial
The Phase 2b ORIGIN clinical trial (NCT04716231) is a global, multicenter, randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of atacicept in 116 patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite being on a stable prescribed regimen of a renin-angiotensin-aldosterone system inhibitor (RAASi) for at least 12 weeks that is the maximum labeled or tolerated dose. The Phase 2b ORIGIN clinical trial evaluated three dose strengths of atacicept versus placebo, administered weekly by prefilled syringe. Patients were randomized 2:2:1:2 to atacicept 150 mg, atacicept 75 mg, atacicept 25 mg, or matching placebo. Upon completion of the 36-week blinded treatment period, all patients were offered open-label atacicept 150 mg for an additional 60 weeks.
The primary endpoint was the change in proteinuria as evaluated by urine protein to creatinine ratio (UPCR) at week 24 and the key secondary endpoint was the change in proteinuria as evaluated by UPCR at week 36. Additional exploratory endpoints include change in proteinuria as evaluated by UPCR at weeks 12, 48, and 96; change in estimated glomerular filtration rate (eGFR); change in serum immunoglobulin levels, and serum galactose-deficient IgA1 (Gd-IgA1) levels; safety and tolerability; and serum pharmacokinetics (PK).
The trial met its primary and key secondary endpoints, with statistically significant and clinically meaningful proteinuria reductions and stabilization of eGFR versus placebo through week 36. The safety profile was comparable between atacicept and placebo.
For more information about the Phase 2b ORIGIN clinical trial, please visit www.clinicaltrials.gov.
About the Phase 3 clinical trial (ORIGIN 3)
The ORIGIN 3 clinical trial (NCT04716231) is a global, multicenter, randomized, double-blind, placebo-controlled Phase 3 trial evaluating the safety and efficacy of atacicept 150 mg in patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite being on a stable prescribed regimen of renin-angiotensin system inhibitors (RASi) (ACEi or ARB) for at least 12 weeks that is the maximum labeled or tolerated dose. The objectives of the trial are to determine the effect of atacicept on proteinuria and preservation of renal kidney function compared to placebo.
The Phase 3 trial is composed of up to a 4-week screening period, a 104-week double-blind treatment period, a 52-week open-label extension and 26 weeks of follow-up. Participants will be randomized 1:1 to atacicept 150 mg once weekly subcutaneous injections (N=188) or placebo once weekly subcutaneous injections (N=188) for 104 weeks, followed by a 52-week open-label extension. The primary endpoint is the change from baseline in proteinuria as evaluated by urine protein to creatinine ratio (UPCR) at week 36. The key secondary endpoint is annualized rate of change in estimated glomerular filtration rate (eGFR) up to week 104. Additional secondary endpoints are the change in Gd-IgA1, change in eGFR up to week 52, and time from randomization to first occurrence of composite kidney failure endpoint event.
For more information about the ORIGIN 3 clinical trial, please visit www.clinicaltrials.gov.
About IgA nephropathy (IgAN), or Berger’s disease
IgAN, also known as Berger’s disease, is a serious and progressive autoimmune disease of the kidney, for which there remains a high unmet medical need. IgAN is driven by the production of immunogenic Gd-IgA1, which triggers autoantibodies that lead to the formation of pathogenic immune complexes, which become trapped in the kidney’s glomeruli, causing inflammation and progressive damage. In up to 50 percent of patients, IgAN can lead to end-stage renal disease (ESRD) or kidney failure, which has considerable morbidity and impact on patients’ lives.
About Atacicept
Atacicept is an investigational recombinant fusion protein that contains the soluble transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) receptor that binds to the cytokines B-cell activating factor (BAFF) and A proliferation-inducing ligand (APRIL). These cytokines are members of the tumor necrosis factor family that promote B-cell survival and autoantibody production associated with certain autoimmune diseases, including IgAN and lupus nephritis. The Phase 2b ORIGIN clinical trial of atacicept in IgAN met its primary endpoint and showed a statistically significant reduction in mean proteinuria versus baseline at weeks 24 and 36. Vera believes atacicept is positioned for best-in-class potential, targeting B cells and plasma cells to reduce autoantibodies and having been administered to more than 1,500 patients in clinical studies across different indications.
About Vera Therapeutics
Vera Therapeutics is a late clinical-stage biotechnology company focused on developing treatments for serious immunological diseases. Vera’s mission is to advance treatments that target the source of immunologic diseases in order to change the standard of care for patients. Vera’s lead product candidate is atacicept, a fusion protein self-administered as a subcutaneous injection once weekly that blocks both B-cell activating factor (BAFF) and A proliferation-inducing ligand (APRIL), which stimulate B cells and plasma cells to produce autoantibodies contributing to certain autoimmune diseases, including IgAN, also known as Berger’s disease, and lupus nephritis. In addition, Vera is evaluating additional diseases where the reduction of autoantibodies by atacicept may prove medically useful. Vera is also developing MAU868, a monoclonal antibody designed to neutralize infection with BK virus (BKV), a polyomavirus that can have devastating consequences in certain settings including kidney transplantation. Vera retains all global developmental and commercial rights to atacicept and MAU868. For more information, please visit www.veratx.com.
Forward-looking Statements
Statements contained in this press release regarding matters, events or results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the ability to execute against strategy for Vera’s programs, atacicept’s potential to be a best-in-class therapy and a disease-modifying treatment for patients with IgAN, the therapeutic potential of atacicept’s dual inhibitor approach to treating the cause of IgAN, Vera’s plans to complete enrollment of the pivotal Phase 3 ORIGIN 3 trial, the design and management of Vera’s clinical trials, and Vera’s product candidates, strategy, and regulatory matters. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believe,” “potential,” “will,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Vera’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks related to the regulatory approval process, results of earlier clinical trials may not be obtained in later clinical trials, preliminary results may not be predictive of topline results, risks and uncertainties associated with Vera’s business in general, the impact of macroeconomic and geopolitical events, and the other risks described in Vera’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Vera undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
For more information, please contact:
Investor Contact:
Joyce Allaire
LifeSci Advisors
212-915-2569
[email protected]
Media Contact:
Mari Purpura
LifeSci Advisors
[email protected]
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/9a2a1f73-36c5-432e-9151-eed0870b3c76


