Beckman Coulter's SARS-CoV-2 IgG Antibody Test Receives FDA Emergency Use Authorization
BREA, Calif., June29, 2020 /PRNewswire/ -- Beckman Coulter today announced that its Access SARS-CoV-2 IgG assay has received Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA).
BREA, Calif., June 29, 2020 /PRNewswire/ -- Beckman Coulter today announced that its Access SARS-CoV-2 IgG assay has received Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA). Beckman Coulter has already shipped tests to more than 400 hospitals, clinics and diagnostics laboratories in the U.S., and has begun distribution of the new antibody test globally to countries that accept the FDA EUA and CE Mark. The company is able to deliver more than 30 million tests a month.
Henry Ford Health System was one of the first health systems to receive Beckman Coulter's test and independently validate its performance.
"We selected the Beckman Coulter Access SARS-CoV-2 IgG antibody assay to be the backbone of Henry Ford's COVID-19 serology testing program because of its outstanding performance in our rigorous independent evaluation," said Dr. Bernard C Cook, Division Head of Chemistry-Pathology, Henry Ford Health System. "Henry Ford found when running the Beckman Coulter SARS-CoV-2 assay on 204 PCR-confirmed COVID-19 patient samples, a test sensitivity of 100% at 14 days post-PCR and testing of 80 patient samples from the pre-COVID era yielded a specificity of 100%."
"At a time when significant confusion was created by the initial influx of poor-quality antibody tests, our team worked meticulously to develop a highly sensitive and specific assay," said Julie Sawyer Montgomery, president of Beckman Coulter. "With 100% Positive Percent Agreement and 99.6% Negative Percent Agreement, our test significantly reduces the risk for false positives, delivering the results that health care providers and their patients can trust. A lot has been written about accuracy issues with the initially launched antibody tests, but a test at this level offers positive predictive values greater than 90% even in very low prevalence communities. And, in areas hardest hit by the virus, the positive predictive values of our assay are greater than 98%.1,2"
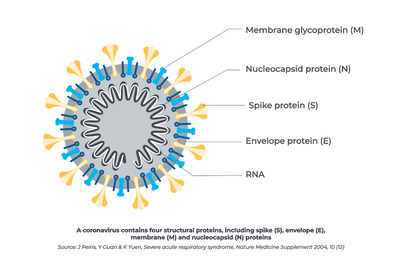
Additionally, of the tests developed by the top four in vitro diagnostic manufacturers capable of delivering high-volume testing to the U.S., Beckman Coulter's test is the only SARS-CoV-2 IgG assay which targets antibodies that recognize the receptor binding domain (RBD) of the spike protein which SARS-CoV-2 uses to bind to a human cell receptor. This is significant as antibodies which target the RBD have the potential to be neutralizing and thus prevent future infection by blocking the virus from entering the cell. It is for this reason many vaccine developers are also targeting the RBD of the spike protein in their vaccine development.3
Beckman Coulter has more than 16,000 immunoassay analyzers worldwide, 3,500 of which are in the United States. Many of Beckman Coulter's analyzers can deliver up to 400 routine tests an hour. A large number of analyzers are connected to hospital information systems, enabling laboratories to automate the reporting of serology test results. The Access SARS-CoV-2 IgG test can also be run on Beckman Coulter's Access 2 analyzer, a compact table-top analyzer enabling high-quality serology testing to be carried out in small hospitals and clinics. This test seamlessly integrates into laboratory workflows making it easy to add serology testing to routine blood tests performed during inpatient and wellness testing. This type of testing can enable health systems to comprehensively determine the immune status of their communities and potentially identify individuals that are eligible for future plasma donation.
"We anticipate that understanding the immune status of communities and convalescent plasma donation will play important roles in the fight against COVID-19 before a vaccine is widely available," said Shamiram R. Feinglass, M.D., MPH, chief medical officer Beckman Coulter. "While there is more to learn regarding how long an individual's immune response to the SARS-CoV-2 virus lasts, this test may be crucial to determining the portion of the population that may already be immune."
Sawyer Montgomery added, "Our assay can be utilized in a variety of healthcare settings, including central laboratories, as well as smaller clinics and hospitals in underserved communities with a range of Beckman Coulter immunoassay analyzers. This accessibility is vital to ensuring all communities, including minority, rural and urban have access to this testing and answers they can trust."
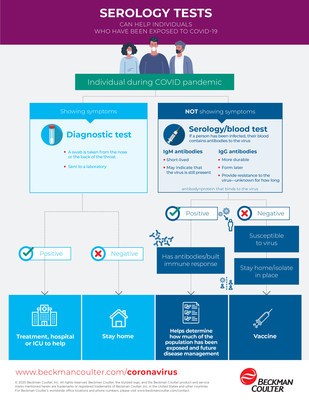
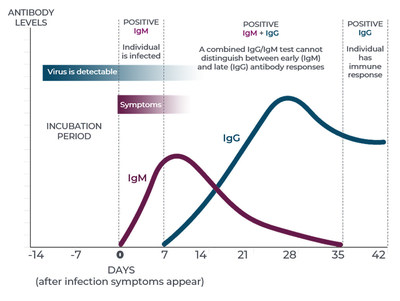
While immunoglobulin M (IgM) antibodies play a prominent role in the body's primary antibody response to infection, they decline within a short timeframe. IgG antibodies begin developing within the first 14 days, and may last for months or years depending upon the pathogen and the individual.
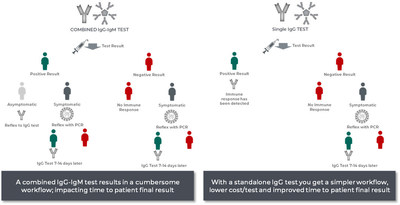
Dr. Feinglass added, "We developed the Access SARS-CoV-2 IgG test to help clinicians determine if a patient was infected with COVID-19 in the past and developed an immune response. In contrast, a total antibody test can't help a clinician determine whether an individual is currently infected or whether they developed an immune response from an earlier infection. The clinician must therefore perform additional testing, requiring added time and cost."
About the Access SARS-CoV-2 IgG Assay
The Access SARS-CoV-2 IgG Assay is a qualitative immunoassay that detects IgG antibodies directed to the receptor binding domain of the spike protein of the novel coronavirus that is driving the ongoing global pandemic. It is believed that these antibodies have the potential to be neutralizing antibodies and may play a role in lasting immunity. The test has a confirmed 100% Positive Percent Agreement (sensitivity) and 99.6% Negative Percent Agreement (specificity) and at 18 days post PCR confirmed positive test. The assay uses immobilized virus antigens on magnetic particles to capture IgG antibodies from patient blood or serum samples and reveals them using labeled anti-IgG antibodies. The Access SARS-CoV-2 IgG assay can be used with a variety of Beckman Coulter analyzers, including the high-throughput DxI 800 designed for large labs, to the DxI 600 for mid-sized labs and the DxCi and Access 2 analyzers for smaller labs and healthcare clinics. The assay can be seamlessly integrated into existing workflows without batch processing.
Beckman Coulter will continue to focus on bringing innovative tests to market, and will be seeking EUA for IL-6 and Access SARS-CoV-2 IgM in the near future. For the latest information on the Access SARS-CoV-2 IgG assay, or for more information about Beckman Coulter's commitment to the fight against COVID-19, visit: www.BeckmanCoulter.com/Coronavirus.
About Beckman Coulter
Beckman Coulter is committed to advancing healthcare for every person by applying the power of science, technology and the passion and creativity of our teams to enhance the diagnostic laboratory's role in improving healthcare outcomes. Our diagnostic systems are used in complex biomedical testing, and are found in hospitals, reference laboratories and physician office settings around the globe. Beckman Coulter offers a unique combination of people, processes and solutions designed to elevate the performance of clinical laboratories and healthcare networks. We do this by accelerating care with a menu that matters, bringing the benefit of automation to all, delivering greater insights through clinical informatics and unlocking hidden value through performance partnership. An operating company of Danaher Corporation (NYSE: DHR) since 2011, Beckman Coulter is headquartered in Brea, Calif., and has more than 11,000 global associates working diligently to make the world a healthier place.
|
Media Resources: |
||
|
• |
Beckman Coulter media kit |
|
|
• |
Whiteboard video and other video assets |
|
|
• |
B-Roll footage of Access SARS-CoV-2 IgG Assay being shipped from Hebron, KY Beckman Coulter and lab footage |
|
|
• |
Infographics, medical illustrations and photos |
|
|
References: |
|
1 At 3.5% prevalence rate, PPV is 90.1%; at 16% prevalence rate PPV is 97.9%. When equivocal results are excluded, a 2% prevalence rate yields a 91.1% PPV and a 16% prevalence rate yields 99% PPV. |
|
2 Koenig, D. Evidence Mounts for Greater COVID Prevalence, WebMD, https://www.webmd.com/lung/news/20200424/more-data-bolsters-higher-covid-prevalence |
|
3 Grifoni, A., et al., Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals, Cell (2020), prepublication, doi: https://doi.org/10.1016/j.cell.2020.05.015 |
|
© 2020 Beckman Coulter. All rights reserved. Beckman Coulter, the stylized logo, and the Beckman Coulter product and service marks mentioned herein are trademarks or registered trademarks of Beckman Coulter, Inc. in the United States and other countries. |
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/beckman-coulters-sars-cov-2-igg-antibody-test-receives-fda-emergency-use-authorization-301084670.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/beckman-coulters-sars-cov-2-igg-antibody-test-receives-fda-emergency-use-authorization-301084670.html
SOURCE Beckman Coulter Diagnostics